Every day new challenges face the American marketplace as COVID-19 continues its unpredictable path. However, even as supply chain disruptions arise and costs for goods climb, consumer purchasing habits are not slowing down. With increased product options across all industries, it is now more important than ever that state leaders prioritize the safety of their constituents. This is particularly vital when it comes to health products and medical devices.
A person’s health is deeply personal and deserves equally individualized attention. But if you’ve noticed that more and more one-size-fits-all medical products have entered the market, you’re not alone. This has been the emerging method to drive costs down and expand access. Unsurprisingly, scientific research shows that for critical health conditions, do-it-yourself options should always be considered a supplement to professional medical oversight. After all, safety should always be the priority.
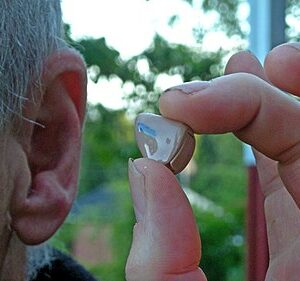
The hearing health industry is currently in the process of introducing a new over-the-counter market for hearing aids, a move that seeks to provide hearing assistance to Americans suffering from perceived mild-to-moderate hearing loss. This process was initiated in 2017 when senators Elizabeth Warren and Chuck Grassley proposed the bipartisan Over-the-Counter Hearing Aid Act. The emergence of COVID-19 stalled its implementation, but thankfully the Biden administration has given this initiative new life. These devices — like all medical devices — require concrete safety standards and regulations.
In the fall of 2021, President Biden called on the Food and Drug Administration to do just that and issue new regulations for OTC hearing devices. These regulations will ensure that any direct-to-consumer hearing devices are safe, effective and properly inspected. Unfortunately, even after the FDA announces its final rule, the guidelines will not take effect until at least mid-year. This delay leaves the industry and consumers in a vulnerable limbo, which is why it is imperative state leaders can enact and enforce consumer protections.
As with all new regulations, the FDA shared its proposal for public comment. During this time, more than 40 state attorneys general shared their concern regarding language that would virtually repeal “all the state-requested exemptions from preemption issued by the FDA since 1980 — even those related exclusively to non-OTC hearing aids.”
These exemptions are necessary in allowing state regulators to continue in their role as primary enforcers of consumer protection laws. All 50 states currently have hearing professional licensing requirements, and many have critical hearing aid consumer protections like mandatory warranties and advertising restrictions. These state-level protections are key in keeping consumers safe.
It is also imperative that federal regulators approach OTC hearing aids as medical devices, not consumer electronics. By allowing over-amplification and unclear age verification processes, these devices could end up in the hands of someone who might ultimately harm their long-term health.
As it stands, there is nothing to prevent manufacturers from combining OTC hearing aids with other wearable consumer technologies. And while enticing for profits, this could expand the use well beyond adults with perceived mild-to-moderate hearing loss. These devices are meant to better American lives, not potentially worsen them.
Studies have found that when it comes to hearing loss, an improper hearing aid can lead to increased chances of dementia or physical injury. “Even a mild degree of hearing loss triples the risk of an accidental fall.” That is why the FDA must ensure OTC hearing aid regulations adequately protect consumers from improper use, or faulty products.
Companies across the nation are deceptively marketing mere amplification devices as hearing aids, and it’s up to regulators — state attorneys general included — to keep them at bay. Indeed, we applaud those state attorneys general who have already taken action to do so. Hearing affects all aspects of a person’s life, which is why those seeking help are particularly vulnerable to deceptive claims promising relief.
As federal regulators weigh the variables needed to assist Americans suffering from mild-to-moderate hearing loss, we urge them to recognize the key role that states play in keeping consumers safe. The introduction of a new OTC market is a welcome opportunity to help more Americans than ever before, but only if their safety remains the priority.